
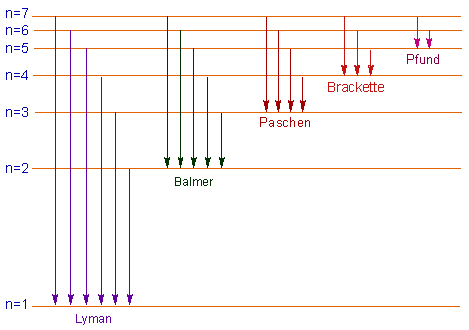
The shortest wavelength/highest energy light (violet 410 nm) causes the electron to jump up four levels, while the longest wavelength/lowest energy light (red 656 nm) causes a jump of only one level. Each of the absorption lines corresponds to a specific electron jump. In the visible part of the spectrum, hydrogen absorbs light with wavelengths of 410 nm (violet), 434 nm (blue), 486 nm (blue-green), and 656 nm (red). The absorption spectrum of hydrogen shows the results of this interaction. (Remember when we said that photons only carry very specific amounts of energy, and that their energy corresponds to their wavelength?) Said in another way, electrons absorb only the photons that give them exactly the right energy they need to jump levels. The energy that an electron needs in order to jump up to a certain level corresponds to the wavelength of light that it absorbs. In addition, it takes a very discrete amount of energy-no more, no less-to move the electron from one particular level to another. The interesting thing is that the electron can move only from one energy level to another. It can jump one level or a few levels depending on how much energy it absorbs. When the atom absorbs light, the electron jumps to a higher energy level (an “excited state”). When a hydrogen atom is just sitting around without much energy, its electron is at the lowest energy level. It consists of a single proton in the nucleus, and one electron orbiting the nucleus. Absorption of Light by HydrogenĪ hydrogen atom is very simple.
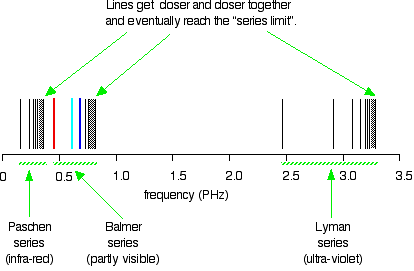
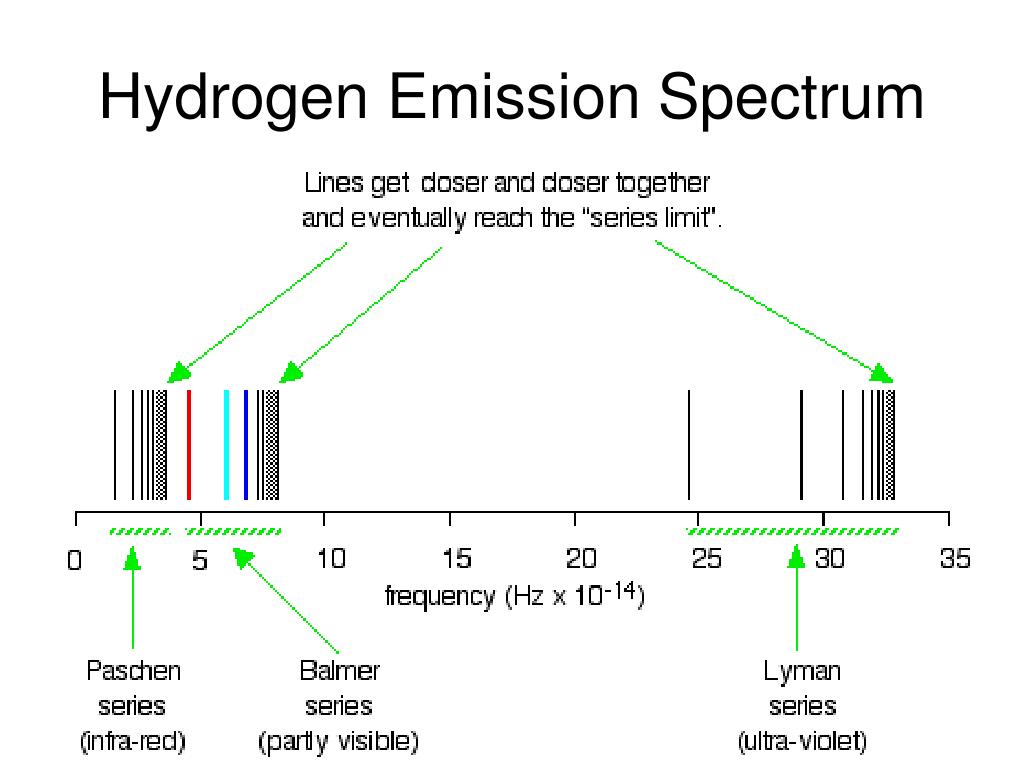
Why is this? Let’s take a look at hydrogen, the most abundant element in the universe. We can do both of these because each element has its own unique spectrum.Īn element’s spectrum is like its fingerprint, its autograph, its barcode. We can use a glowing nebula’s emission spectrum to figure out what gases it is made of based on the colors it emits. We can use a star’s absorption spectrum to figure out what elements it is made of based on the colors of light it absorbs.
A comparison was also made between direct current and radiofrequency powered glow discharges and very similar trends have been obtained.Let’s go back to simple absorption and emission spectra. On the other hand, it has been found that nitrogen does not have any similar effect: the gradient of the intensity ratios of both elements is negligible or even negative and no emission lines are enhanced in the presence of nitrogen. It has been also observed that this effect is more pronounced at lower currents. Furthermore, in the case of iron, several emission lines with the excitation energy between 5.3 and 5.6 eV are strongly enhanced in the presence of hydrogen. In the case of hydrogen, when intensity ratios (intensities measured in argon–hydrogen relative to those measured in pure argon) are plotted against the excitation energies of the lines, these intensity ratios increase with the excitation energy between approx. The results presented in this paper focus on the effects of hydrogen and nitrogen on intensities of atomic emission lines of iron and titanium. Therefore, it is important to describe these effects in detail and to try to understand the processes involved. These changes, caused by the molecular gases which are very often present in the discharge for various reasons, have a serious impact on the accuracy of analytical results. It is now well known that traces of molecular gases such as hydrogen or nitrogen can affect significantly the electrical characteristics, sputtering rates and relative intensities of emission lines in glow discharge optical emission spectrometry ( GD-OES).


 0 kommentar(er)
0 kommentar(er)
